The fuel cell stack is the heart of a fuel cell power system. Hydrogen is fed to one electrode and oxygen to the other.

How Does A Hydrogen Fuel Cell Work Akio Tv Youtube
In contrast to other electric vehicles FCEVs produce electricity using a fuel cellpowered by hydrogen rather than drawing electricity from only a battery.

. Heres how a fuel cell produces electricity. Fuel cells do not have to be recharged. Therefore these cells can constantly generate electricity until the supply of fuel and oxygen is cut off.
They can use a wide range of fuels and feedstocks and can provide power for systems as large as a utility power station and as. In a fuel cell hydrogen energy is converted directly into electricity with high efficiency and low power losses. A fuel cell is a device that generates electricity through an electrochemical reaction not combustion.
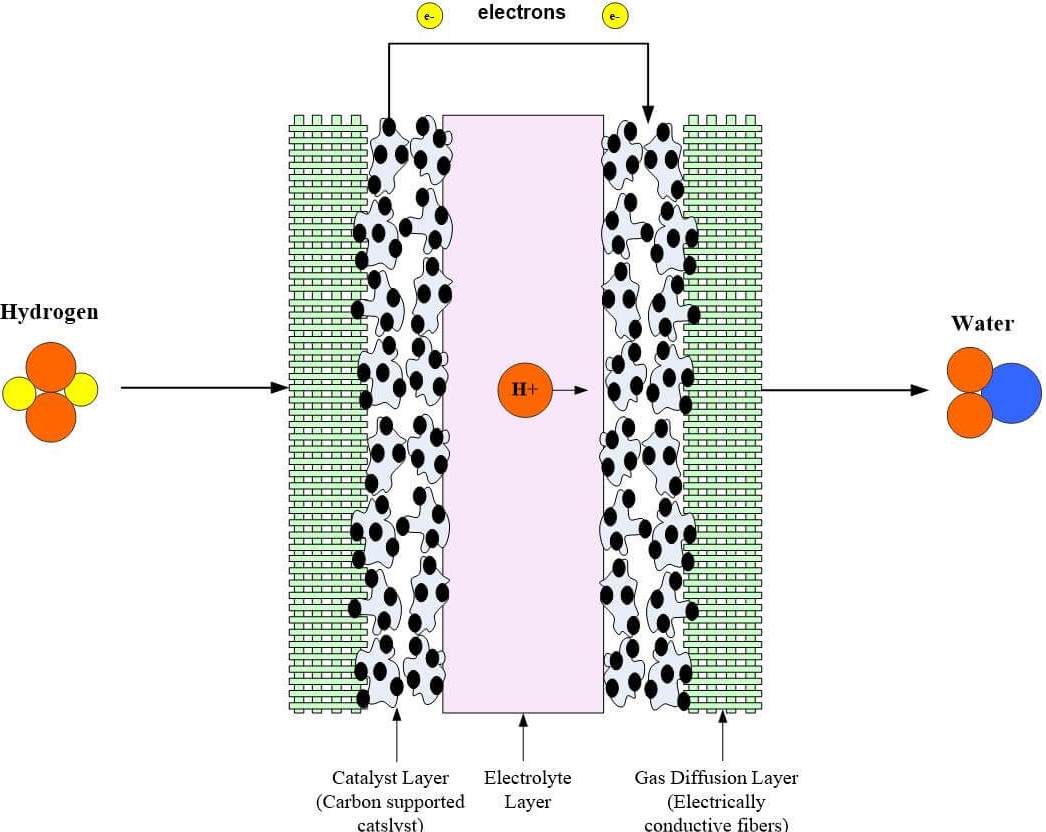
Fuel cells cleanly and efficiently convert chemical energy from hydrogen-rich fuels into electrical power and high-quality heat via an electrochemical process that is efficient and emits water rather than pollutants as there is no burning of the fuel. These cells require a continuous input of fuel and an oxidizing agent generally oxygen in order to sustain the reactions that generate the electricity. The diagram and animation below show how a PEM fuel cell works.
A fuel cell resembles a battery in many respects but it can supply electrical energy over a much longer period of time. Similar to how an internal combustion engine needs a. Oxygen from the air big turquoise blobs comes down a second pipe to the negative terminal.
This conversion of fuel is radically advanced over the typical gas generator available to home boat and RV owners. The heart of a PEM fuel cell is the membrane electrode assembly MEA which includes the membrane the catalyst layers and gas diffusion layers GDLs. But how does it actually power a vehicle.
The proton from the hydrogen migrates through the electrolyte to the oxygen. A fuel cell is a device that generates electricity by a chemical reaction. How Fuel Cells Work.
The reactants flow in and products flow out while the electrolyte remains in the cell. Like all-electric vehicles fuel cell electric vehicles FCEVs use electricity to power an electric motor. Hydrogen therefore is an energy carrier which is used to move store and deliver energy produced from other sources.
A fuel cell can be defined as an electrochemical cell that generates electrical energy from fuel via an electrochemical reaction. In a fuel cell hydrogen and oxygen are combined to generate electricity heat and water. This can be achieved by either traditional internal combustion engines or by devices called fuel cells.
Describe how a fuel cell works it uses energy from the reaction of a fuel with oxygen to create a voltage. Fuel cells consist of two electrodes that conduct electrons the anode and the cathode. Polymer Electrolyte Membrane PEM fuel cells used in automobilesalso called Proton Exchange Membrane fuel cellsuse hydrogen fuel and oxygen from the air to produce electricity.
Similar to a battery a fuel cell is comprised of many individual cells that are. Hydrogen is the fuel of tomorrow. A typical gas generator inefficiently burns fuel to rotate an engine and directly coupled alternator.
Although fuel cells and batteries are both considered electrochemical cells and consist of similar structures fuel cells require a continuous source of fuel and oxygen to run. How a fuel cell works. How Fuel Cells Work.
They produce electricity and heat as long as fuel is supplied. A WATT fuel cell converts fuel into power by oxidizing fuel via electrochemical conversion. During the vehicle design process the vehicle manufacturer defines the power of the vehicle by the size of the electric motors.
Fuel cells work like batteries but they do not run down or need recharging. This is because a fuel cell is continuously supplied with fuel and air or oxygen from an external source whereas a battery contains only a limited amount of fuel material and oxidant that are depleted with use. Hydrogen gas from the tank shown here as big brown blobs feeds down a pipe to the positive terminal.
In a battery the electrical energy is chemically stored in the electrode. If hydrogen is the fuel the only products are electricity water and heat. A single fuel cell produces less than 1 V which is insufficient for most applications.
The reactions that produce electricity take place at the electrodes. Fuel cells are used today in a range of applications from providing power to homes and businesses keeping critical facilities like hospitals grocery stores and data centers up and. It generates electricity in the form of direct current DC from electrochemical reactions that take place in the fuel cell.
In a fuel cell the energy source is brought in from externally. It is exactly the opposite of electrolysis. A fuel such as hydrogen is fed to the anode and air is fed to the cathode.
A fuel cell uses the chemical energy of hydrogen or other fuels to cleanly and efficiently produce electricity. Hardware components used to incorporate an MEA into a fuel cell include gaskets which provide a seal around the MEA to prevent leakage of gases and bipolar plates which are used to assemble individual PEM fuel. A fuel-cell generates electricity by combining hydrogen with oxygen.
A fuel cell consists of two electrodesa negative electrode or anode and a positive electrode or cathodesandwiched around an electrolyte. The electrodes are separated by an electrolyte that conducts ions. Fuel cells provide usable electrical and heat energy directly by eliminating efficiency.
Fuel cells are unique in terms of the variety of their potential applications. Hydrogen is flammable and explosive so the tank has to be extremely strong. Fuel cells can operate virtually continuously so long as the fuel and oxygen continue to flow into the cell.
Hydrogen atoms are split in a fuel cell and electricity is produced with the elec. A fuel cell is a device that generates electrical power through a chemical reaction by converting fuel hydrogen into electricity. Every fuel cell has two electrodes called respectively the anode and cathode.

A Basic Overview Of Fuel Cell Technology

0 Comments